Newsletter
Streamlining GxP Compliance with Celito’s Continuous Software Assurance Framework
Mani Madhuri
Manager,
Computer Systems Validation

Bhanu Sharma
Senior Manager,
Computer Systems Validation

In today’s fast-paced life sciences environment, maintaining compliance with GxP regulations is essential for ensuring patient safety, product quality, and data integrity. Celito’s Continuous Software Assurance (CSA) framework is designed to streamline this process for GAMP Category 4 applications by following a comprehensive approach rooted in “risk-based” decision-making and “critical thinking.” This approach is aligned with the FDA’s recent draft guidance, which emphasizes identifying intended use, applying a risk-based methodology, and selecting the appropriate assurance activities to ensure compliance.
Celito’s CSA framework builds on the foundational principles of GAMP5 and the FDA’s latest guidance, offering organizations a more efficient way to manage software releases and upgrades while staying compliant. By reducing the complexity of traditional validation processes, organizations can more easily focus on delivering quality products and meeting regulatory requirements.
Celito’s Continuous Software Assurance Framework:
Computer System Validation (CSV) performed by software development companies and life sciences industry has been a point of discussion for various CSV professionals as companies and business functions like to minimize the time spent on documentation with reference to article published in 2021 by ISPE: Special Interest Group: CSA Whitepaper for Industry Implementation and Compliance Group; CSA | FDA | Computer Software Assurance Risk-Based Approach.
Celito’s framework can help companies establish a robust and leaner assurance strategy, which will save time on documentation. Here’s how the Celito’s framework works:
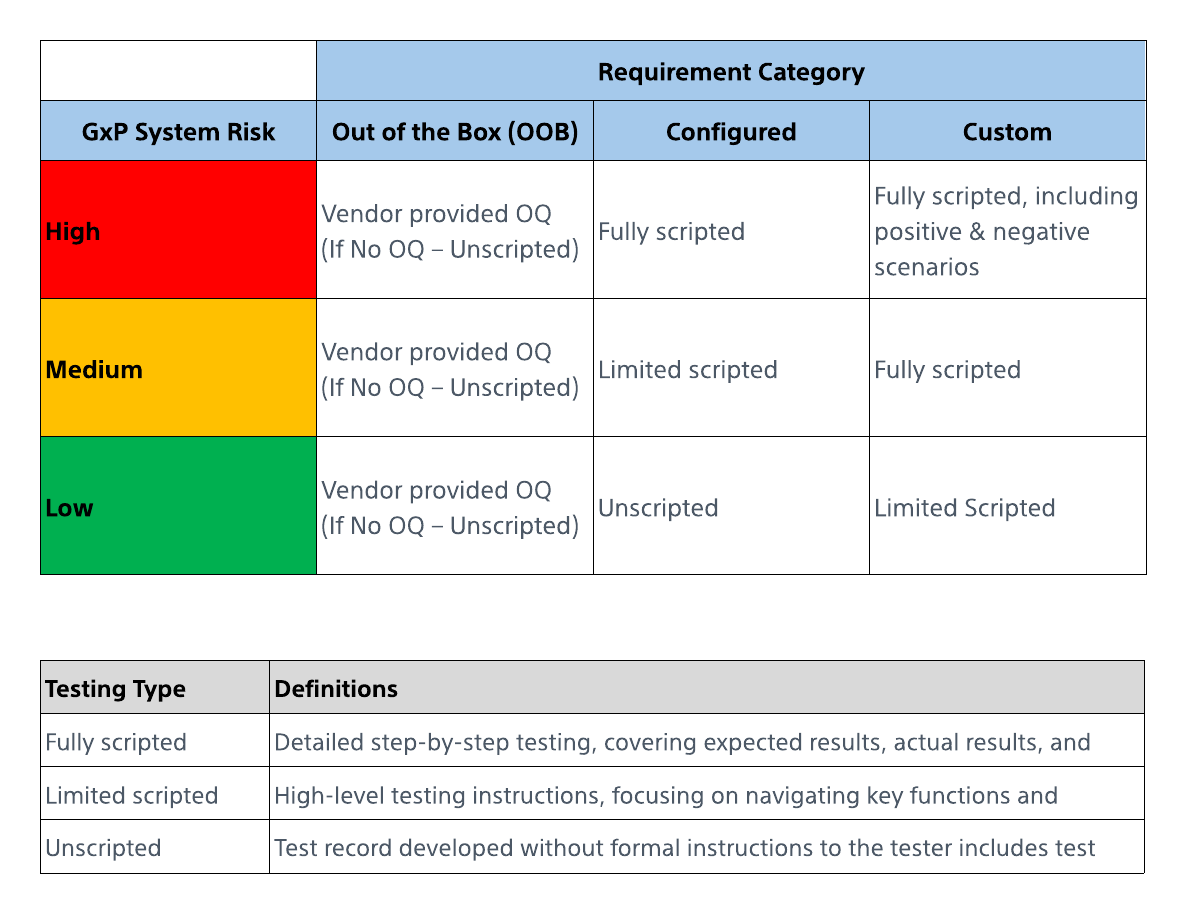
1. GxP Risk Assessment:
The first step is to perform a comprehensive risk assessment, focusing on three key factors—patient safety, product quality, and data integrity. Systems are classified into three risk levels:
- Low Risk: No direct impact on GxP intended use, patient safety, product quality, or data integrity.
- Medium Risk: Indirect impact on GxP intended use, patient safety, product quality, or data integrity.
- High Risk: Direct impact on GxP intended use, patient safety, product quality, or data integrity.
2. Requirements Categorization:
For GAMP Category 4 (Configurable) systems, the requirements are categorized into:
- Out of the Box (OOB)
- Configured
- Customized
With these two foundational steps, the CSA Testing Matrix ensures that 80% of efforts are dedicated to testing and only 20% to documentation. This balance ensures that compliance is achieved without unnecessary administrative overhead.
Celito can assist clients with applying the CSA framework for release management activities. Implementing the CSA framework allows for effective management and assessment of software releases, considering their category (Major/Minor) and impact on intended use or business processes.
Conclusion:
The CSA risk framework is a forward-thinking approach that aligns with the FDA’s evolving guidance. Celito’s CSA framework helps organizations reduce documentation time while maintaining a validated state for their GxP systems by focusing on risk-based decisions and critical thinking. This streamlined approach ensures that software releases are compliant, efficient, and fully aligned with the latest regulatory standards, allowing teams to focus on what truly matters.
References:
- Computer Software Assurance for Production and Quality System Software
Draft guidance for FDA Staff, issued on September 13, 2022.

Celito is a team of experienced IT Executives, Industry Professionals, and Business Consultants focused on the life sciences industry.
Products
Consulting
Company
Celito Tech, Inc.
CORPORATE HEADQUARTERS
2100 Geng Road Suite #210
Palo Alto, CA 94303
CALIFORNIA OFFICE
842 Main St.
Redwood City, CA 94063
+1 650.374.2121
FLORIDA OFFICE
1221 Brickell Avenue Suite #900
Miami, FL 33131
Celito Tech, Inc.
INDIA OFFICE
Celito Tech India Pvt Ltd.
5th Floor,
Regus Grandeur Offices Pvt Ltd,
Caddie Commercial Tower, Aerocity
New Delhi 110037, India
+91 987.011.6939
Privacy Policy | Cookie Policy | Terms of Service | Copyright 2021 © Celito Tech, Inc.