Protecting Biotech Success: The Crucial Role of
NIST CSF in Safeguarding Drug Development
and Clinical Innovation
Introduction
Technology is rapidly changing and evolving across industries at a speed that allows malicious threat actors to take advantage of lapsed security measures put into place by organizations. In the life sciences sector this is no exception, with the potential for protected health information (PHI) to be compromised, it is crucial for life sciences organizations to implement and evolve a robust cybersecurity program that follows industry standards and adheres to the latest in security best practices. That is where the National Institute of Standards and Technology’s (NIST) Cybersecurity Framework (CSF) plays a vital role in defining and implementing an organization’s cybersecurity strategy.
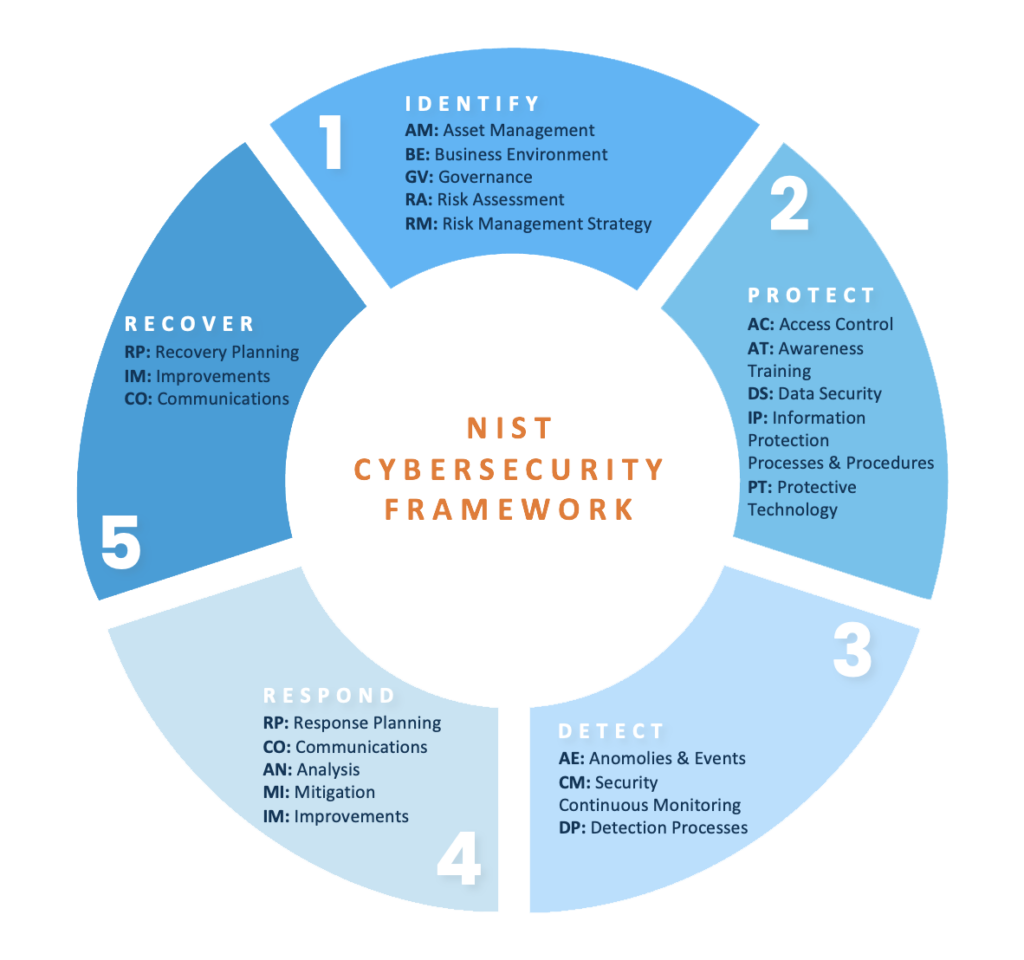
What is the NIST CSF?
The NIST CSF is a set of guidelines and best practices that is published by the U.S. Government, designed to help organizations prioritize and manage risks related to cybersecurity. The NIST CSF contains just over one hundred controls that focus on cybersecurity governance, the implementation of security tooling and technologies, and the process for effectively and efficiently responding to security incidents. To accomplish this holistically, NIST has implemented five control areas in version 1.1 of their framework: Identify, Protect, Detect, Respond, and Recover. The framework allows for organizations to perform self-assessments to track progress against achieving maturity on the outlined controls through the implementation of policies, procedures, and technologies. When assessing an organization under the NIST CSF guidelines, it is recommended to use a maturity model such as the five levels of the Capability Maturity Model Integration (CMMI) to accurately track progress and control maturity over time.
How does NIST CSF help to protect Biotech organizations?
The many aspects of security and governance that are outlined in the controls of NIST CSF directly tie into regulatory requirements and industry best practices surrounding cybersecurity at life sciences companies. In particular, the following items are crucial to an organization’s cybersecurity posture and are covered thoroughly throughout NIST CSF controls in all five control areas.
- Regulatory Compliance: Due to the highly regulated nature of the biotech industry, NIST CSF provides a structured set of controls and implementation recommendations that align with regulatory requirements from bodies such as the Health Insurance Portability and Accountability Act (HIPPA), General Data Protection Regulation (GDPR), and the Securities and Exchange Commission (SEC), which have all implemented stringent cybersecurity requirements that organizations must follow.
- Protecting Sensitive Data: Depending on the stage of a biotech organization, they may house and maintain sensitive personally identifiable information (PII) and protected health information (PHI) about patients and clinical trials. Adhering to the controls within NIST CSF surrounding data encryption and classification, as well as the proper handling and backups of sensitive data will aid companies in ensuring the best practices around sensitive data protection.
- Business Continuity and Risk Management: A large portion of the NIST CSF controls outline an approach to organizational risk management that is proactive and iterative, which allows organizations to properly identify and mitigate risks before they have the potential to become incidents and breaches that impact the ability for business to function. In addition to internal risk management, CSF also contains controls surrounding third party risk management and continuous evaluation recommendations that enable biotech organizations to quantify the risk of a vendor or partner before intertwining them into the company.
Conclusion
The NIST Cybersecurity Framework is a useful, data-driven tool that helps to guide organizations on implementing best practices and ensuring regulatory compliance in the areas of cybersecurity and governance. By conducting periodic self-assessments based on a maturity-based model, biotech companies can track a moment-in-time view of where their cybersecurity posture stands. The NIST Cybersecurity Framework is one of the most widely utilized and understood cybersecurity frameworks within the life sciences industry, and directly correlates implementation efforts to control maturity.
Think your organization would benefit from a NIST CSF assessment?
At Celito, we have industry leaders and cybersecurity experts who are ready to lead your organization through a preliminary NIST CSF assessment, plan remediation steps to get your organization to your goal and execute on those remediation plans to further your organization’s cybersecurity posture and ensure regulatory cybersecurity compliance.
External Links:
- National Institute of Standards and Technology, Cybersecurity Framework v1.1. (2018). Published cybersecurity framework and supporting controls to enhance organizational cybersecurity posture and governance.
- Capability Maturity Model Integration (CMMI). (2024). Outlines the five major levels of maturity that can help evaluate the maturity of an entire organization or its pieces.
- National Institute of Standards and Technology, Cybersecurity Framework. (2024). Published by the United States Government, the NIST Cybersecurity Framework aims to provide cybersecurity best practices to guide organizations on building a secure and robust cybersecurity posture.